Growing cell cultures in the laboratory is a common procedure in biology. For some years now, researchers have been able to grow cell cultures for much longer periods than before. Thus, small three-dimensional structures can be created in the laboratory which have a tissue organisation resembling that of human organs. These structures can even imitate some functions of organs. It is because of this partial similarity to human organs that they are called organoids.
Organoids that contain tissue structures from nerve and glial cells typical of brain tissue are called brain organoids. Researchers hope to obtain many new insights into the development and function of the human brain from research using these brain organoids. However, some people have ethical concerns about this kind of research. Questions such as the following come up in this context: To what extent does a brain organoid resemble a human brain? Can a brain organoid perceive what is happening to it? Can it even develop consciousness? Or at least feel pain?
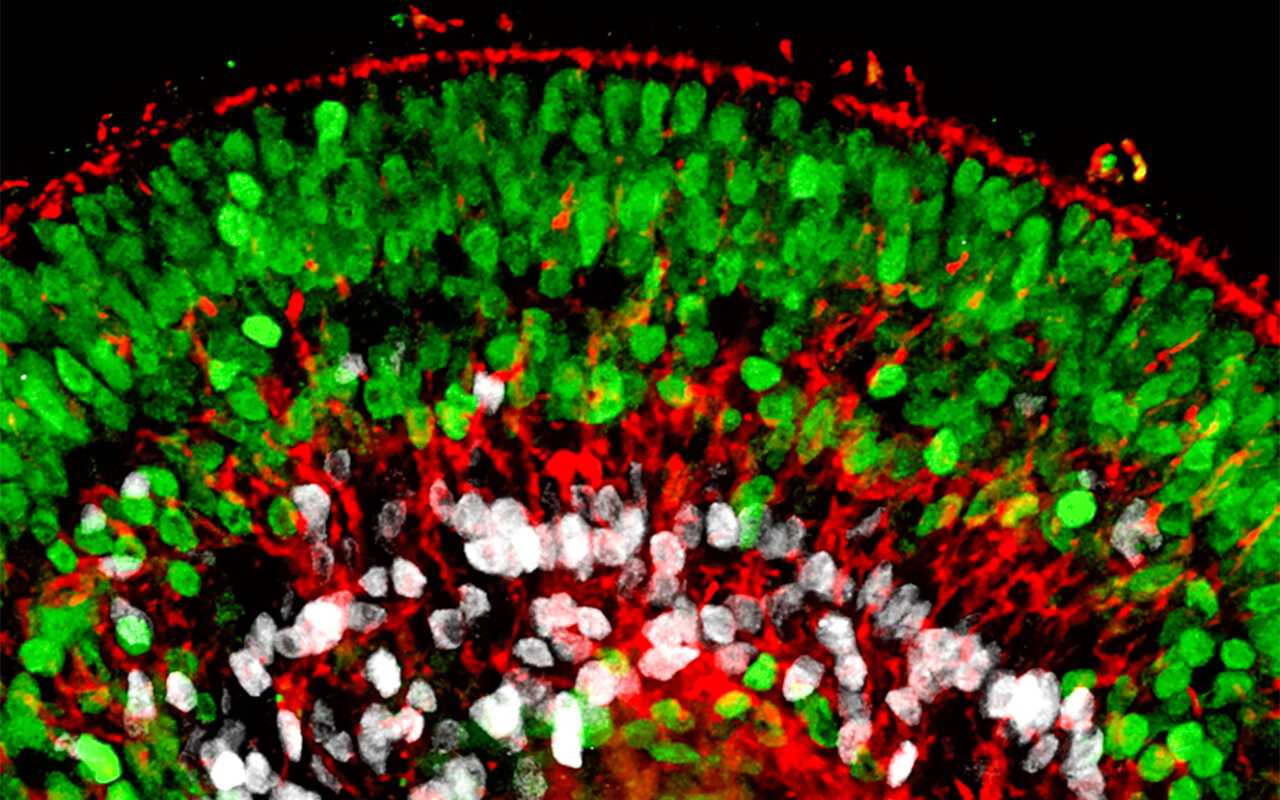
The German National Academy of Sciences Leopoldina answers these questions in this digital dossier. The dossier introduces current research on and with brain organoids, shows opportunities and possibilities for future research, and discusses ethical and legal aspects. The dossier was prepared on the basis of a statement written by an interdisciplinary working group of researchers, who reviewed the current state of research in summer 2022.
How the human brain works
The human brain performs a number of impressive tasks. It allows us to perceive our environment and move within it, remember the past, imagine the future and communicate. At the same time, it controls our bodily functions. It regulates our body temperature as well as the intake of fluids and nutrients and mobilises additional energy reserves in times of stress.
The brain has developed into a highly complex organ to perform these many functions. Compared to other animals, such as primates, the human cerebral cortex is considerably larger and much more folded. It also contains certain areas that do not exist in any other species. It is assumed that this enlarged cerebral cortex makes human thought and language possible.
All brain areas are made up of similar cell types. The brain consists of approximately 85 billion nerve cells, organised into different brain structures. Each individual cell can form over 1,000 connections with other nerve cells. Thus, the adult brain has almost 100 trillion connections. Information is processed primarily by nerve cells, also called neurons. These are connected to one another by synapses which transmit neuronal signals.
However, many questions concerning the brain have so far not been answered:
How exactly does the human brain work?
How do psychological diseases develop?
How do drugs work in the brain?
How does the human brain develop during pregnancy and after birth?
In what conditions do neurodegenerative diseases develop?
However, getting to the bottom of these questions can be problematic: performing research on the brain of a living person would be unethical in many cases. Furthermore, many research questions cannot be answered without studying certain factors in isolation and in a controlled environment. As the human brain contains several unique structures, animal experiments only provide limited learning opportunities.
This is where brain organoids provide new possibilities for research.
Brain organoids
Researchers can influence the growth of brain organoids so that the cells either organise themselves (into a brain organoid with several different brain regions) or so that brain region-specific organoids are formed, e.g. retinal, midbrain or forebrain organoids.
Brain organoids are in a sense a “cell-biological window” that offers insights into human brain development and the development of neuronal diseases. They allow the study of interactions between cells and their environment. For example, the effect of drugs or the influence of various toxins, germs and viruses on human brain cells and brain development can be studied with the help of brain organoids.
A specific example of a practical application is an analysis of the high incidence of microcephaly in connection with Zika virus infections. Brain organoid models were used to demonstrate the causal link between a Zika virus infection and the development of microcephaly, and to decode the underlying mechanisms and test possible drug treatments.
How are brain organoids formed?
Like all organoids, brain organoids are produced from stem cells. Brain organoids consist of nerve and glial cells that are derived from embryonic stem cells (ES cells) or induced pluripotent stem cells (iPS cells).

Due to certain growth factors, either brain region-specific organoids (e.g. retinal, midbrain or forebrain organoids) or brain organoids consisting of several of these structures can develop.
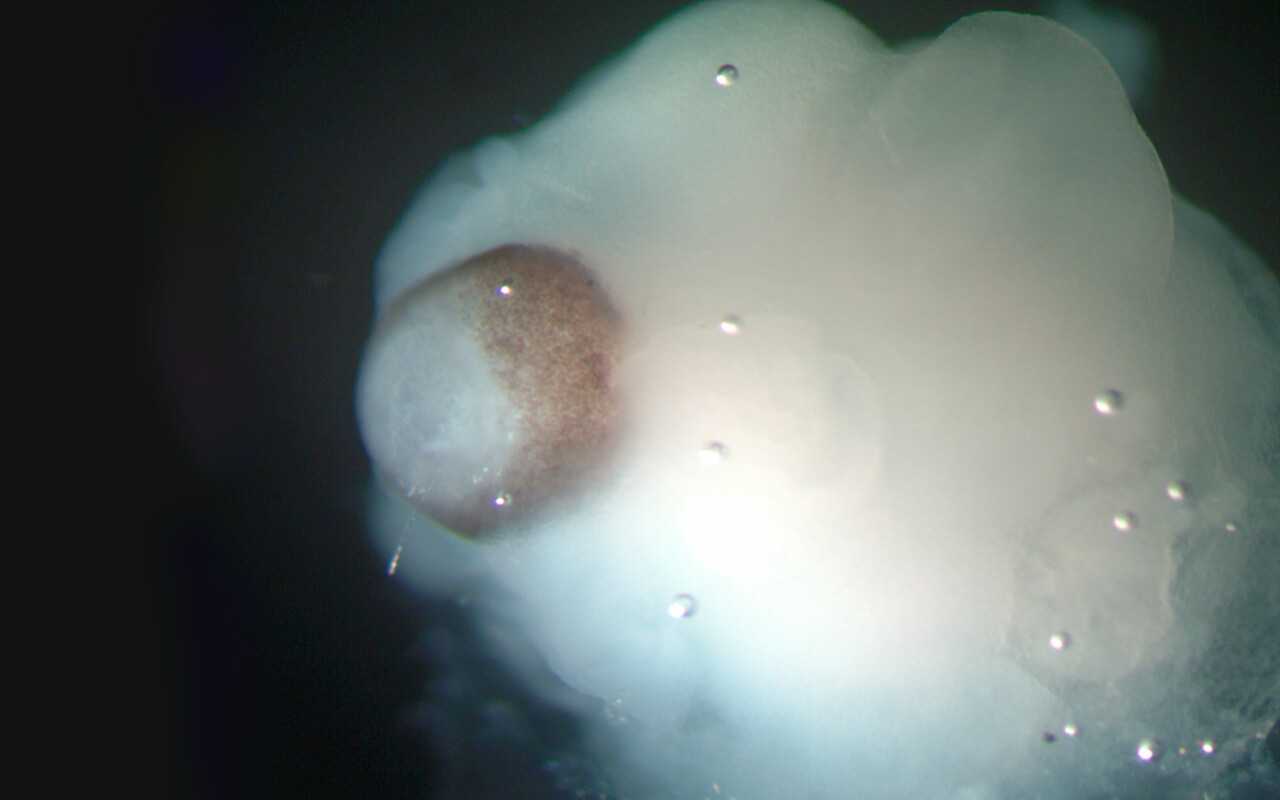
The process of brain organoid development is very time-consuming. The culture and development processes correspond to the time scale of human development. This means that over the course of weeks and months, cells divide into organoids and specialise, thereby forming complex structures.
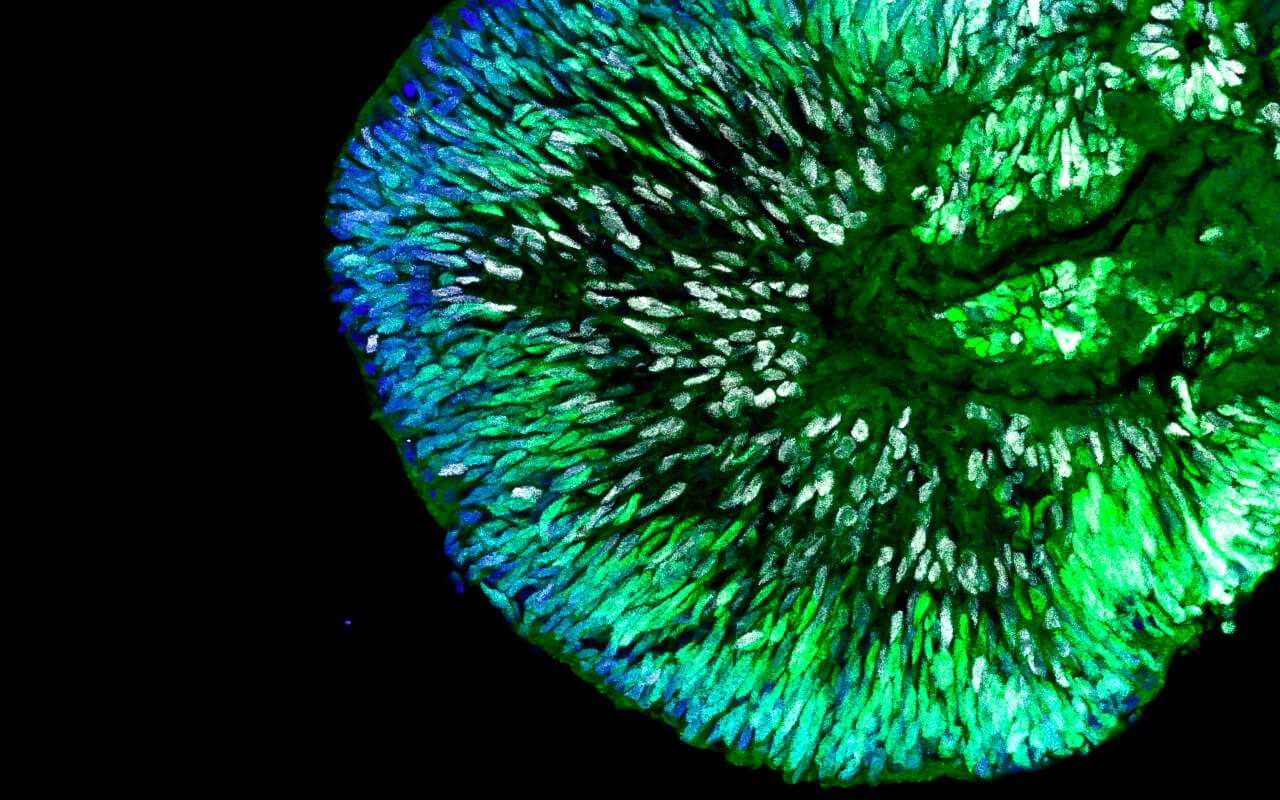
Thus, although early developmental stages can be partially modelled, it has not yet been possible to model later developmental stages. This is mainly because brain organoids do not form blood vessels. Without a vascular system and a stable blood and nutrient supply, the growth and developmental ability of brain organoids are severely limited.

Brain organoids, as used in research today, are usually no larger than a pea and can be cultivated in the laboratory for several weeks to months.
Currently, basic developmental processes and functional mechanisms of the human brain are not well understood – and thus important details for better understanding and treating neurological, psychiatric or neurodegenerative diseases are missing. Although many functions of the human brain can be studied on animal models, there are fundamental properties that are unique to human brain function and can therefore only be replicated in human models.
The following are some examples of the opportunities for research with brain organoids:

Better understanding the development of the human brain
Brain organoids can be used to study the principles and mechanisms of human brain development. They can be used to understand the development of a healthy brain. They are also suitable for observing and understanding the formation of developmental disorders.
At the moment, brain organoids only reach the maturity level of prenatal brains. Early malformations of the brain can thus be studied effectively with the brain organoid model. However, the current models are not adequate for many disorders and diseases that develop later in life, such as neurodegenerative diseases. This is because the induced pluripotent stem cells used for brain organoids lose the biological age characteristics of their original cells during the reprogramming in preparation for organoid development.

Understanding diseases of the brain
Brain organoids help research the causes of brain diseases, in particular complex neuropsychiatric diseases. Many of these diseases, e.g. epilepsy or autistic spectrum disorders, are associated with mutations in individual genes and/or disrupted brain development.
By using brain organoids produced from a patient’s own stem cells, the effects of mutations on brain development can be observed at the cellular, physiological and molecular levels. This makes it possible to study causes of disease and develop possible therapies, potentially for individualized therapies.
For diseases suspected to be caused by single mutations, brain organoids in combination with new methods of gene editing, such as the so-called “gene scissors” CRISPR-Cas9, make it possible to directly test this assumption. In this case a mutation which is thought to be linked to a disease is corrected in patient stem calls or also introduced into stem cells from healthy control subjects. Brain organoids are then produced from these stem cells and their development is compared. This shows whether the mutations are the actual cause of the respective disease.
However, most brain diseases have complex causes in which environmental and genetic risk factors work together. In such a case, the presence of many gene variants, which are often also found in healthy people, lead to an increased risk of disease only when combined with other factors.
Brain organoids allow us to study not only living, developing brain cells in the cell network, but also their reactions to possible environmental risk factors, including prenatal infections, toxins or stress hormones. Genetic and environmental risks often have to combine to trigger a disease. This can be studied in brain organoids and leads to insights which cannot be obtained from animal models (where the genetic risks are not the same as for humans) or from post-mortem human tissue (which no longer reacts to the environment).
Testing drugs and other substances
The brain is particularly sensitive during its early development, for example to toxins, drugs or medicines. Brain organoids make it possible to study the effects of such substances on brain development. This could facilitate the search for early therapies or preventative approaches. Brain organoids are also used to identify new drugs for certain neuropsychiatric diseases, so far mainly Parkinson’s, Alzheimer’s and autism.
Brain organoids could also help us understand why certain drugs lead to an improvement or cause side effects in some but not all patients. This can be achieved by comparative studies of brain organoids from patients who respond to certain therapy and “therapy-resistant patients”.
What can brain organoids not (yet) do?
Although brain organoids are an important building block for the study of brain diseases, they cannot yet map many aspects relevant to disease, at least not very well. They should therefore be used in combination with other methods. An example is the study of disrupted communication between different areas of the brain, which is a central element of many psychiatric diseases. Here, “assembloids”, which model the interaction of different regions of the brain, promise new advances in knowledge. Organoids from different brain areas are assembled for this.
Brain organoids are also used to help us understand neurodegenerative diseases, such as Alzheimer‘s or Parkinson’s, and develop new therapies. However, some pathological changes only occur after many years of life. Brain organoids are so far most comparable to the foetal brain. Methods that accelerate the ageing of organoids or illustrate the context of a disease on an aged brain could be of assistance here.
Brain organoids as a model for the effects of a Zika virus infection
Zika virus, endemic in some parts of the world and mainly transmitted by mosquitoes of the Aedes genus, causes “Zika fever” in humans.
In the majority of cases, symptoms of the disease are mild. However, epidemiological data from recent Zika virus epidemics has suggested that there is a causal relationship between a Zika virus infection during pregnancy and impaired brain development in the foetus.
Children with this malformation have a noticeably small head circumference, referred to as microcephaly.
Exposing a developing brain organoid to the Zika virus results in similar developmental disorders.
Research on brain organoids has not only uncovered the causal links between the virus infection and impaired brain development but has also helped identify suitable active substances for use as drugs in potential therapies.
What ethical questions should be discussed?
Some people have voiced ethical concerns after learning about research on brain organoids, for exaple through media reports. After all, this research involves cellular tissue that forms the biological substrate of our mind which is used in an extremely artificial manner. Frequent questions include, for example, whether brain organoids could develop at least a rudimentary form of consciousness or feel pain.
Some of the ethical concerns surrounding these questions are discussed in the following.
Do brain organoids have consciousness?
The short answer is no. This is due to a number of reasons. According to current research, a number of conditions have to be met for consciousness to develop. The biological structures involved in the formation of consciousness need to be of sufficient size, complexity and differentiation. The development of consciousness also requires the cooperation of many, functionally specialised centres in the brain and the precisely coordinated interactions of nerve cells in their development. Information provided by processes in different regions of the brain has to be combined. Nerve networks have to be kept in a critical, very finely regulated state of excitation. As they develop through sensory-mediated interaction with the environment, the brain’s connectivity architecture needs to adapt to allow the formation of higher cognitive functions. The developmental steps of the organism and its nervous system are finely coordinated and mutually dependent.
Only by being embedded in the organism and through interactions with the environment do brains acquire the abilities which we assume are based on conscious processing. Brain organoids, in the form in which they can be produced at present, have neither the necessary size and complexity, nor are they connected to sensory organs or embedded in an organism with which they develop in parallel. They can therefore neither experience nor develop states of consciousness, however rudimentary.
The question of whether this could change in future if one day larger and more complex brain organoids could be produced cannot be conclusively answered based on the current level of knowledge.
Do brain organoids feel pain or other stimuli?
The sensation of pain is a complex process that needs cells with pain receptors and requires and involves a number of different brain areas. Pain receptors are located on nerve endings at the periphery of the body (such as in the skin). On being stimulated, their signals travel to neurons in the spinal cord. Even if such nerve cells with pain receptors could be coupled to brain organoids, this does not mean that organoids can also feel pain. This is because, for now and in the foreseeable future, they lack the necessary prerequisites, in particular the presence and interconnection of different brain areas.
The same applies to visual stimuli. Vision is a complex process that requires associative brain areas that are able to interpret and derive meaning from received stimuli. The formation or presence of certain receptors is therefore only a very small part of this process.
Do brain organoids have a moral status?
Over the past decade ‘moral status’ has become a key concept in bioethics. Moral status is used to decide which moral rights an entity has, e.g. a human being, an animal or an object. Possession of a moral status is primarily understood to be a characteristic fully attributed to born humans, but not at all to inanimate things or products. Anything that lies between is assigned widely varying value.
The attribution of moral status is often linked to the respective entity having subjective interests. The more complex these interests are, the weightier the moral status becomes. For example, it can be assumed that a pain-sensitive being will have an interest in avoiding pain. Thus, it should then have the right to avoid pain, and this right may only be contravened for more important reasons.
Considerations of the moral status of brain organoids so far have been developed primarily from the viewpoint of existing attributions of status for animals and humans at various stages of development – for example embryos created in the laboratory.
Previous considerations on possible ethical claims for the protection of brain organoids have mainly been directed towards the possibility of determining the capacity for consciousness in future. If brain organoids were ever able to feel pain, a majority of ethicists would grant them the right to avoid these sensations.
Does the moral status change when brain organoids are transplanted into another organism?
A significant limit to the formation of very mature cell types and complex neuronal circuits is imposed by the conditions of their cultivation in the laboratory. A lack of blood vessels restricts the supply of nutrients, particularly in the interior of brain organoids, and thus also limits their growth. Brain organoids cultivated over a long period increasingly lose their three-dimensional organisation, i.e. the correct topographical arrangement of different cell types.
However, human brain organoids can be integrated into different host organisms at different developmental stages. This integration creates a more natural developmental environment that supports a longer growth period and a better nutrient supply, especially by means of the vascular system of the host organism. The transplantation of human brain organoids into a living organism has so far been performed on mice, rats and macaques. There are a few natural limitations to such experiments. For one, expansion of the human brain organoids is limited by the space available. And mice, for example, have a maximum life span of three years.
However, there are possible scenarios that would allow a very long period of maturation in donor organisms. One such scenario would be the transplantation of brain organoids into the brain of a large, long-living organism. In such an organism, for example a domestic pig or a primate, brain organoids could develop over many years and would have much more space to expand. It is likely that brain organoids would be fully vascularised in such organisms and that there would be intensive connections of nerve cells between the host and organoid.
The transplantation of a brain organoid into a host organism raises different ethical issues and could under certain conditions affect the moral status of the host organism (see previous question).
Vascularisation in vitro would avoid these problems. Research into this is already taking place. One approach is to combine mechanical pump systems with assembloids of brain and blood vessel organoids. Another approach is to produce blood vessels in vitro and combine them with brain organoids. However, even using this approach, it has not yet been possible to integrate a closed, functioning blood vessel system into a brain organoid.
Do brain organoids have rights?
German law distinguishes between legal subjects and legal objects. A human being as a legal subject can be the bearer of rights and obligations. Legal objects, e.g. things, can be assigned to the person by the legal system so that rights can apply to them. However, they have no rights of their own. For example, animals are legal objects in Germany.
In the case of new entities such as brain organoids, it needs to be decided first of all whether they are “legal subjects” or “legal objects”. However, assigning an entity to the category of “legal object” does not mean that this entity does not have any legal protection. The German Constitution (Basic Law) for example grants animals and the environment a right to protection, without at the same time granting them their own rights.
The body of a living human being is not a legal object, in particular it is not a thing capable of being owned. But when bodily substances are separated from the body, property law is applicable. They can now be considered to be property. If “a new movable thing” has been produced by “processing or transforming one or more substances [of the original cells]”, the researcher that has produced them acquires ownership. This also applies if the new thing is an organoid.
In general, it is therefore the case that brain organoids are legal objects, not legal subjects.
Conclusion
Brain organoids provide new opportunities for better understanding the basic developmental processes and functional mechanisms of the human brain. In the foreseeable future, research on and with brain organoids in vitro will not raise any ethical or legal issues requiring regulation. The conditions under which human cells can be used to generate brain organoids are also already sufficiently regulated.
The current limits to the potential development of brain organoids could possibly be overcome in future thanks to developments in this field of research. In this case, established procedures of self-regulation within the scientific community should be used to assess and react to ethically, legally or socially relevant developments in this field at an early stage.
Further information can be found in the statement “Brain organoids – model systems of the human brain”, on which this digital dossier is based. Download it here:

